P. Chedraui, L. Hidalgo, G. San Miguel, N. Morocho, S. Ross
Red clover extract (MF11RCE®) supplementation and postmenopausal vaginal and sexual health
Published in International Journal of Gynecology and Obstetrics (2006)
Int J Gynaecol Obstet. 2006 Dec;95(3):296-7. Epub 2006 Sep 27
Number of patients: 53
| Study Setting | MF11RCE® Group | Control Group |
| Inclusion Criteria | amenorrhea > 12 months and a basal FSH > 30 mIU/mL), > 40 years, non-hormone therapy users, with a basal Kupperman index ≥15 | amenorrhea > 12 months and a basal FSH > 30 mIU/mL), > 40 years, non-hormone therapy users, with a basal Kupperman index ≥15 |
| Exclusion Criteria | positive pregnancy test, nonwillingness, on hormonal therapy (HT), known isoflavone hypersensitivity | positive pregnancy test, nonwillingness, on hormonal therapy (HT), known isoflavone hypersensitivity |
| Parameter | Body mass index and vaginal/sexual health related indicators (dyspareunia, vaginal dryness and decreased libido, graded 0 to 3) were assessed at baseline, 90 and 180 days. At equal intervals vaginal cytologic sampling was performed for the determination of the karyopyknotic, cornification and basal cell maturation index. | Body mass index and vaginal/sexual health related indicators (dyspareunia, vaginal dryness and decreased libido, graded 0 to 3) were assessed at baseline, 90 and 180 days. At equal intervals vaginal cytologic sampling was performed for the determination of the karyopyknotic, cornification and basal cell maturation index. |
| Treatment | MF11RCE® 80 mg for a 90-day period. After a 7 day washout period, subjects switched to receive the opposite | Placebo for a 90-day period. After a 7 day washout period, subjects switched to receive the opposite |
Introduction
Approximately 75% to 85% of postmenopausal women seek medical attention due to symptoms of vaginal atrophy and atrophic vaginitis (Pandit L at al., 1997). 72.7% of postmenopausal women have been found to present vaginal dryness associated to decreased libido and sexual avoidance (Chedraui P et al., 2005).
Aim of the Study
The aim of the study was to evaluate the effect of MF11RCE® supplementation over vaginal and sexual health.
Materials and Methods
The women randomly assigned to one of two groups: either 80mg MF11RCE® per day for a 90 day period, or placebo of equal design. After a 7 day washout period, medication was crossed-over for another 90 days. Body mass index and vaginal/sexual health related indicators (dyspareunia, vaginal dryness and decreased libido, graded 0 to 3) were assessed at baseline, 90 and 180 days. At equal intervals vaginal cytologic sampling was performed for the determination of the karyopyknotic, cornification and basal cell maturation index.
Patients & Study Design
A prospective randomized, double-blind, placebo-controlled cross over trial was carried out to evaluate the effect of MF11RCE® supplementation over vaginal and sexual health of sixty postmenopausal women (amenorrhea >12 months and a basal FSH > 30 mIU/mL), > 40 years, non-hormone therapy users, with a basal Kupperman index ≥15.
Statistical Analysis
Statistical analysis was performed with EPI-INFO 2000 statistical software (Centers for Disease Control, Atlanta, Ga., USA). Comparison of continuous and categorical data was performed with paired t student test and chi-square respectively. A p-value of < 0.05 was considered as significant.
Results
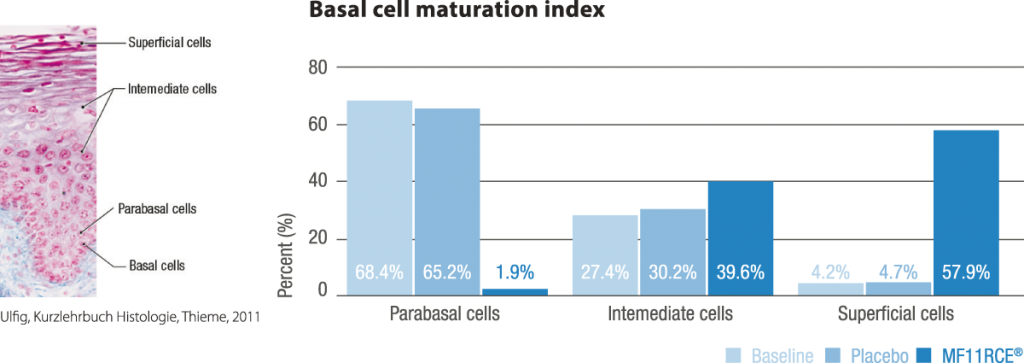
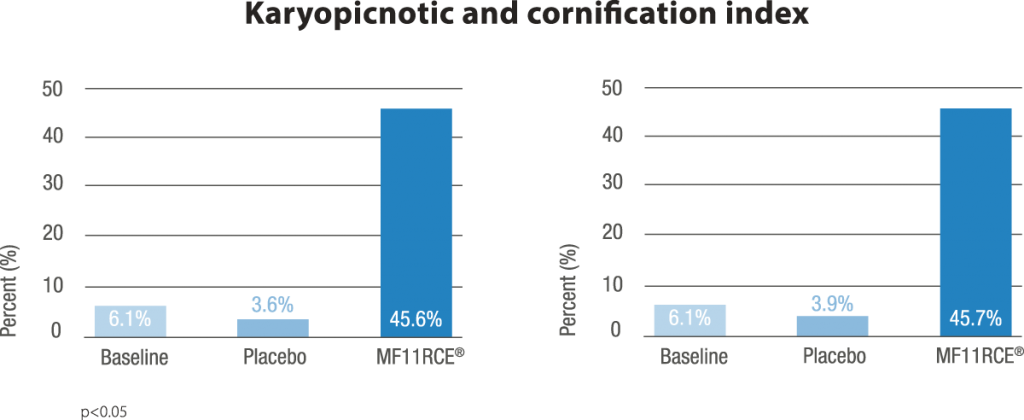
MF11RCE® supplementation exerted a positive effect over the vagina which was evidenced by a significant improvement of the karyopyknotic, cornification and basal cell maturation index. This was correlated with a decrease in the rate of women having dyspareunia, vaginal drynes and decreased libido. Additionally the percentage of subjects presenting moderate to severe scorings within the latter mentioned parameters also decreased significantly. In climacteric women in terms of vasomotor symptoms, bone mineral density, mood, vaginal and sexual health, and serum lipids. Despite this, RC data on skin, appendages, including mucosal sites, are scarce or lacking.
Conclusion
In the present study, compared to placebo, MF11RCE® supplementation had a significant beneficial effect over vaginal and sexual health in postmenopausal women supporting its use as an alternative treatment of postmenopausal vaginal atrophy.
Practical Benefits
In postmenopausal women with vaginal atrophy, MF11RCE® supplementation may
- improve vaginal epithelium’s cell maturation, and
- thus, induce improvement of vaginal atrophy, especially in elder women with increased risk for
cardiovascular events and/or breast cancer,- alleviate vaginal dryness,
- decrease dyspareunia,
- increase libido and allow improvement of sexual life, and
- thus, improve quality of life in menopause.